Articles from Cognito Therapeutics

Cognito Therapeutics, a pioneering neurotechnology company developing disease-modifying therapies for neurodegenerative diseases, today announced new results from a post hoc analysis of its OVERTURE feasibility clinical trial showing that its investigational therapy, Spectris AD™, significantly slows the progression of Alzheimer’s disease.
By Cognito Therapeutics · Via Business Wire · June 11, 2025

Cognito Therapeutics, a pioneering neurotechnology company developing disease-modifying therapies for neurodegenerative diseases, today announced new data from its OVERTURE feasibility trial at the AD/PD™ 2025 International Conference on Alzheimer’s and Parkinson’s Diseases. The results showed that Spectris™, the company’s non-invasive investigational neuromodulation device, maintains corpus callosum structure and evokes gamma oscillation responses that correlate with baseline brain structure in patients with mild-to-moderate Alzheimer’s disease.
By Cognito Therapeutics · Via Business Wire · April 2, 2025

Cognito Therapeutics, a pioneering neurotechnology company developing disease-modifying therapies for neurodegenerative diseases, today announced it will present new clinical data from its OVERTURE study at the AD/PD™ 2025 International Conference on Alzheimer’s and Parkinson’s Diseases, taking place April 1-5, 2025, in Vienna, Austria.
By Cognito Therapeutics · Via Business Wire · March 25, 2025

Cognito Therapeutics, a pioneering neurotechnology company developing disease-modifying therapies for neurodegenerative diseases, today announced key executive appointments designed to accelerate the company’s growth, clinical advancement, and commercialization. Steve Worthy joins as Chief Business & Financial Officer, Dr. Robbert Zusterzeel as Chief Clinical & Regulatory Officer, Deanna Angello as Chief Commercial Officer, and Pritesh Shah, Pharm.D., as Chief Strategy Officer.
By Cognito Therapeutics · Via Business Wire · February 12, 2025

Cognito Therapeutics, a neurotechnology company advancing disease-modifying therapies to treat CNS diseases, today announced the publication of a study in Alzheimer's & Dementia: Translational Research & Clinical Interventions that highlights myelin and synaptic biomarker changes in Alzheimer’s patients treated with its non-invasive medical device, Spectris™.
By Cognito Therapeutics · Via Business Wire · February 11, 2025

Cognito Therapeutics, a neurotechnology company advancing disease-modifying therapies to treat CNS diseases, presented early-stage cost-effectiveness analysis of its Spectris™ therapy for Alzheimer’s disease (AD) at the International Society for Pharmacoeconomics and Outcomes Research Europe (ISPOR Europe) 2024 Annual Meeting in Barcelona, Spain.
By Cognito Therapeutics · Via Business Wire · November 19, 2024

Cognito Therapeutics, a neurotechnology company advancing disease-modifying therapies to treat CNS diseases, presented new data today at the 17th Clinical Trials on Alzheimer’s Disease (CTAD) conference demonstrating that Spectris™, a disease-modifying treatment, significantly reduced the Alzheimer’s Disease Dependence Score in patients with mild to moderate Alzheimer’s disease. The findings were reported from the Phase 2 OVERTURE study, which highlights the potential of Spectris™ in improving patient outcomes and reducing caregiver burden.
By Cognito Therapeutics · Via Business Wire · October 29, 2024

Cognito Therapeutics, a leader in technology-based therapeutic interventions for neurodegenerative diseases, today announced a publication demonstrating structural brain preservation in Frontiers in Neurology. The paper, titled “Spectris™ Treatment Preserves Corpus Callosum Structure in Alzheimer’s Disease,” highlights the potential of Cognito’s Spectris™ as a disease-modifying candidate for Alzheimer’s disease (AD).
By Cognito Therapeutics · Via Business Wire · October 16, 2024

Cognito Therapeutics, a neurotechnology company advancing disease-modifying therapies to treat CNS diseases, announced today it has been selected to present multiple poster presentations for its disease-modifying therapy Spectris™ in patients with Alzheimer’s Disease, at the 17th Clinical Trials on Alzheimer’s Disease (CTAD) conference taking place in Madrid, Spain, and online, October 29 – November 1, 2024.
By Cognito Therapeutics · Via Business Wire · October 7, 2024

Cognito Therapeutics, a leader in technology-based therapeutic interventions for neurodegenerative diseases, today announced that its Board of Directors has appointed Christian Howell as Chief Executive Officer, succeeding Brent Vaughan, who has been pivotal in establishing Cognito as a leader in neurotechnology since his appointment in 2020.
By Cognito Therapeutics · Via Business Wire · August 26, 2024

Cognito Therapeutics, a leader in technology-based therapeutic interventions for neurodegenerative diseases, today announced results from the OVERTURE I study (NCT03556280) which showed that Spectris AD™ significantly slowed the decline in daily functioning and cognitive abilities over six months compared to sham treatment in participants with Alzheimer’s Disease (AD).
By Cognito Therapeutics · Via Business Wire · July 29, 2024

Cognito Therapeutics, a leader in technology-based therapeutic interventions for neurodegenerative diseases, today announced that it will present four scientific posters at the Alzheimer’s Association International Conference 2024 (AAIC). The largest international meeting dedicated to advancing dementia science, AAIC will be held in Philadelphia, PA, and online from July 28 – Aug 1, 2024.
By Cognito Therapeutics · Via Business Wire · July 8, 2024

Cognito Therapeutics, a leader in technology-based therapeutic interventions for neurodegenerative diseases, announced today a poster presentation at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 2024 Meeting, held May 5-8 in Atlanta, GA. The presented research is a targeted literature review of the clinical, humanistic, and economic burden of Alzheimer’s Disease (AD).
By Cognito Therapeutics · Via Business Wire · April 29, 2024

Cognito Therapeutics, a leader in technology-based therapeutic interventions for neurodegenerative diseases, announced today treatment results from OVERTURE II, an open-label extension (OLE) study of Spectris™, a potential disease modifying therapy for the treatment of Alzheimer’s Disease. The results were presented at the 2024 American Academy of Neurology in Denver, CO.
By Cognito Therapeutics · Via Business Wire · April 15, 2024

Cognito Therapeutics, a leader in technology-based therapeutic interventions for neurodegenerative diseases, announced today that data from the open-label extension (OLE) study of Spectris™, a potential disease modifying therapy for the treatment of Alzheimer’s Disease, will be presented at the American Academy of Neurology (AAN) 2024 Annual Meeting, held April 13-18, 2024, in Denver, CO.
By Cognito Therapeutics · Via Business Wire · March 13, 2024

Cognito Therapeutics, a leader in technology-based therapeutic interventions for neurodegenerative diseases, announced today results from the OVERTURE open label extension (OLE) study presented in an oral session at the AD/PD™ 2024 International Conference on Alzheimer’s and Parkinson's Disease. The results support for Spectris’™ potential as a disease modifying therapy that, if approved, could also be used as an early intervention for the treatment of Alzheimer’s Disease (AD).
By Cognito Therapeutics · Via Business Wire · March 11, 2024

Cognito Therapeutics, a leader in technology-based therapeutic interventions for neurodegenerative diseases, announced today the publication of the OVERTURE study results in Frontiers in Neurology. The paper titled "Safety, Tolerability and Efficacy Estimate of Evoked Gamma Oscillation in Mild to Moderate Alzheimer’s Disease," highlights the effects of Spectris, Cognito’s proprietary disease-modifying therapy designed to preserve brain structure and function by evoking gamma frequency oscillations for patients with Alzheimer’s disease (AD).
By Cognito Therapeutics · Via Business Wire · March 6, 2024

Cognito Therapeutics, a leader in technology-based therapeutic interventions for neurodegenerative diseases, announced today it has been selected to present data from an open label extension study evaluating its potential novel therapy, Spectris, in Alzheimer’s Disease (AD), as an oral presentation at the AD/PD™ 2024 International Conference on Alzheimer’s and Parkinson’s Disease, held March 5-9, 2024 in Lisbon, Portugal.
By Cognito Therapeutics · Via Business Wire · February 28, 2024

Cognito Therapeutics, a neurotechnology company developing disease-modifying therapies for neurodegenerative diseases, announced today the first patient enrolled in a biomarker substudy within the HOPE pivotal trial. The HOPE study is evaluating a novel disease-modifying therapy that elicits gamma frequency brain activity through non-invasive sensory stimulation and has the potential to slow the progression of Alzheimer’s Disease, from the comfort of a patient’s home.
By Cognito Therapeutics · Via Business Wire · January 23, 2024

Cognito Therapeutics, a neurotechnology company developing disease-modifying therapies for neurodegenerative diseases, announced today publication of MRI imaging data from its Phase 2 OVERTURE study in the Journal of Alzheimer’s Disease.
By Cognito Therapeutics · Via Business Wire · December 11, 2023

Cognito Therapeutics, a late-stage neuroscience company developing disease-modifying therapies to treat CNS diseases, today announced that Brent Vaughan, Chief Executive Officer, will present at the 35th Annual Piper Sandler Healthcare Conference, being held November 28-30, 2023 in New York, NY.
By Cognito Therapeutics · Via Business Wire · November 13, 2023

Cognito Therapeutics, a neurotechnology company advancing disease-modifying therapies to treat CNS diseases, today announced the appointment of Christian Howell as Chief Commercial Officer (CCO).
By Cognito Therapeutics · Via Business Wire · October 30, 2023

Cognito Therapeutics, a late-stage developer of disease-modifying therapies to treat neurodegenerative disorders, announced today continued active treatment in the Phase 2 OVERTURE open label extension (OLE) study demonstrated durability and concordance of clinical and MRI benefits over 18 months in patients with Alzheimer’s disease (AD).
By Cognito Therapeutics · Via Business Wire · October 24, 2023

Cognito Therapeutics, a neurotechnology company advancing disease-modifying therapies to treat CNS diseases, announced today it has been selected to present clinical data as a late-breaker abstract from its proprietary investigational disease-modifying therapy in patients with Alzheimer’s Disease, at the 16th Annual Clinical Trials on Alzheimer’s Disease (CTAD) conference taking place in Boston and online, October 24-27, 2023.
By Cognito Therapeutics · Via Business Wire · October 18, 2023

Cognito Therapeutics, a pioneer developing disease-modifying therapeutics to treat CNS diseases, today announced the appointment of Greg Weaver as Chief Financial Officer (CFO), to lead all finance-related and investor relations functions at the Company.
By Cognito Therapeutics · Via Business Wire · October 17, 2023

Cognito Therapeutics, a neurotechnology company advancing disease-modifying therapies to treat CNS diseases, today announced robust lobe-specific changes in white matter volume and myelination in Alzheimer’s Disease (AD) patients following six-months of non-invasive gamma stimulation therapy using the at-home wearable device.
By Cognito Therapeutics · Via Business Wire · July 17, 2023

Cognito Therapeutics, a neurotechnology company advancing disease-modifying therapies to treat CNS diseases, today announced that it will present four scientific abstracts at the Alzheimer’s Association International Conference 2023 (AAIC). The largest international meeting dedicated to advancing dementia science, AAIC will be held in Amsterdam, Netherlands and online from July 16-20, 2023.
By Cognito Therapeutics · Via Business Wire · June 26, 2023

Cognito Therapeutics, a neurotechnology company advancing disease-modifying therapies to treat CNS diseases, announced today two poster presentations at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 2023 Meeting, held May 7-10 in Boston, MA. The presented research demonstrates the use of real-world data to identify high-risk mild cognitive impairment (MCI) patients to develop a predictive model for patients at high-risk of progression to AD.
By Cognito Therapeutics · Via Business Wire · May 8, 2023

Cognito Therapeutics, today announced executive leadership team appointments with Ralph Kern, M.D., as Chief Medical Officer and Kim Kwan as Chief Technology Officer who has been promoted from VP of Engineering.
By Cognito Therapeutics · Via Business Wire · April 17, 2023

Cognito Therapeutics, today announced neuroimaging results evaluating the effects of the company’s non-invasive neuromodulation medical device, which delivers proprietary gamma frequency light and sound stimulation in participants with Alzheimer's disease (AD). The results were presented at the AD/PD™ 2023 International Conference on Alzheimer's and Parkinson's Diseases and related neurological disorders, on March 28–April 1, 2023 in Gothenburg, Sweden.
By Cognito Therapeutics · Via Business Wire · March 30, 2023

Cognito Therapeutics, a neurotechnology company advancing disease-modifying therapies to treat CNS diseases, announced today a $73M Series B financing round led by FoundersX Ventures with participation from all existing investors. New investors Alzheimer’s Drug Discovery Foundation (ADDF), Starbloom Capital, IAG Capital and WS Investment Company (Wilson Sonsini’s venture arm) joined the round. The Series B funding brings the total amount raised since inception to $93 million.
By Cognito Therapeutics · Via Business Wire · March 22, 2023

Cognito Therapeutics, a clinical-stage neurotechnology company, announced today the first patient enrollment in its US pivotal study (HOPE), designed to demonstrate the safety and efficacy of its proprietary non-invasive stimulation device CogTx-001 in patients with Alzheimer’s Disease.
By Cognito Therapeutics · Via Business Wire · February 21, 2023

Cognito Therapeutics, a clinical-stage company pioneering disease-modifying therapeutic interventions for neurodegenerative diseases, announced today it has been selected to present clinical data from its proprietary gamma sensory stimulation in patients with Alzheimer’s Disease, at the 2022 Clinical Trials in Alzheimer's Disease (CTAD), held November 29 - December 2, in San Francisco, CA.
By Cognito Therapeutics · Via Business Wire · October 31, 2022
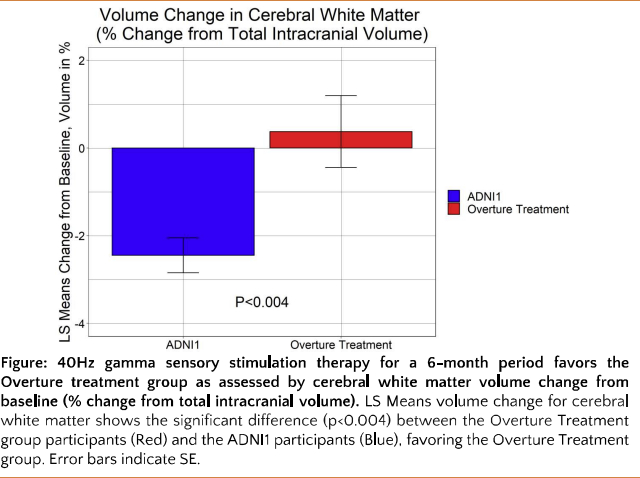
Cognito Therapeutics, announced today that its proprietary gamma sensory stimulation at 40Hz over a 6-month period reduced white matter atrophy in the brain for patients with Alzheimer’s Disease, according to new data presented at the Alzheimer’s Association International Conference 2022.
By Cognito Therapeutics · Via Business Wire · August 1, 2022

Cognito Therapeutics, a pioneer in developing disease-modifying therapeutic interventions for neurodegenerative diseases, today announced the acceptance of two research abstracts and an oral presentation at the Alzheimer’s Association International Conference® 2022 (AAIC®). The largest international meeting dedicated to advancing dementia science, AAIC will be held as an online and in-person event in San Diego, July 31- August 4, 2022.
By Cognito Therapeutics · Via Business Wire · July 13, 2022

Cognito Therapeutics, a pioneer in developing disease-modifying therapeutic interventions for neurodegenerative diseases, today announced it has joined the Digital Medicine Society’s (DiMe) Alzheimer’s Disease and Related Dementias (ADRD) Digital Measures Development Project to support the advancement of its late-stage disease-modifying therapeutics pipeline for neurodegenerative diseases. The company joins other DiMe partners including Biogen, Eli Lilly, Eisai, Alzheimer's Drug Discovery Foundation (ADDF), and many others.
By Cognito Therapeutics · Via Business Wire · May 9, 2022

Cognito Therapeutics and Providence Health Plan today announced a collaboration to conduct a Phase 3 study of Cognito’s breakthrough, non-invasive disease-modifying therapy for patients with Mild Cognitive Impairment (MCI).
By Cognito Therapeutics · Via Business Wire · January 27, 2022

Cognito Therapeutics, a pioneer in non-invasive optogenetics to treat neurodegenerative diseases, announced today the appointment of Jennifer Newberger as Vice President, Head of Regulatory Affairs and Compliance. In this role, Jennifer will lead key regulatory, compliance and operations activities at Cognito in conjunction with the leadership team.
By Cognito Therapeutics · Via Business Wire · December 14, 2021

Digital Therapeutics Alliance (“DTA” or “Alliance”), a global non-profit trade association of industry leaders and stakeholders with the mission of broadening the understanding and adoption of digital therapeutics (DTx) into healthcare, today announced the election of Everett Crosland, Chief Commercial Officer, Cognito Therapeutics to its Board of Directors.
By Cognito Therapeutics · Via Business Wire · December 8, 2021

Cognito Therapeutics, a pioneer in non-invasive neuromodulation to treat neurodegenerative diseases, announced today its CEO Brent Vaughan will speak at CODE Conference, in Beverly Hills, CA, September 27-29, 2021 to discuss Cognito’s ground-breaking technology that has shown the promise of slowing or stopping the progression of Alzheimer’s and its potential to fundamentally expand human cognitive performance.
By Cognito Therapeutics · Via Business Wire · September 15, 2021

Cognito Therapeutics, a Phase 3-ready disease-modifying therapeutics company using non-invasive neuromodulation to reduce or halt the progression of neurodegenerative diseases, announced today the formation of a first-in-category Payor Advisory Board to enable day one payor coverage upon approval and insure timely and broad patient access to Cognito’s breakthrough treatment for Alzheimer’s.
By Cognito Therapeutics · Via Business Wire · September 8, 2021

Cognito Therapeutics, a Phase 3-ready disease-modifying digital therapeutics company developing a new category of therapeutic treatments for neurodegenerative diseases starting with Alzheimer’s Disease, today announced that Brent Vaughan, CEO, will participate in a fireside chat at the BTIG Biotechnology Conference 2021 on Monday, August 9, 2021 from 11:00-11:25 am ET.
By Cognito Therapeutics · Via Business Wire · August 4, 2021

Cognito Therapeutics, a Phase 3-ready neuro-physiology company developing a new class of disease-modifying digital therapeutics to treat neurodegenerative disorders, presented key findings from clinical research involving its lead digital therapeutic candidate at the Alzheimer’s Association International Conference® 2021 (AAIC®), virtual and in-person in Denver, Colorado, July 26-30, 2021.
By Cognito Therapeutics · Via Business Wire · July 26, 2021

Cognito Therapeutics, a clinical-stage company developing a new class of disease-modifying digital therapeutics targeting neuronal pathophysiology to treat neurodegenerative disorders, today announced the acceptance of six research abstracts at the Alzheimer’s Association International Conference® 2021 (AAIC®). The largest international meeting dedicated to advancing dementia science, AAIC will be held as an online and in-person event in Denver, Colorado, July 26-30, 2021.
By Cognito Therapeutics · Via Business Wire · July 14, 2021

Cognito Therapeutics, a Phase 3-ready disease-modifying digital therapeutics company, today announced the appointment of Gerald Chan, ScD. as Chairman of the Board. Cognito is a pioneer in using neuromodulation to engage novel targets of neuronal pathophysiology to achieve disease modification in neurodegenerative diseases. Dr. Chan is the co-founder of Morningside, a privately held venture capital and private equity firm. Since inception, Morningside has focused on the development and commercialization of novel scientific discoveries in both the life sciences and technology sectors.
By Cognito Therapeutics · Via Business Wire · July 6, 2021

Cognito Therapeutics, a clinical-stage company leading the development of a new class of disease-modifying digital therapeutics to treat neurodegenerative disorders including Alzheimer’s disease, announced today its plans to add a second digital therapeutic to the company’s pipeline, initiating a new study evaluating digital therapeutic for individuals who have the genetic rare disease, Down syndrome associated Alzheimer’s Disease (DS-AD).
By Cognito Therapeutics · Via Business Wire · June 14, 2021

Cognito Therapeutics, a clinical-stage company leading the development of a new class of disease-modifying digital therapeutics to treat neurodegenerative disorders including Alzheimer’s disease, announced today the appointment of Everett Crosland as Chief Commercial Officer. In this role, Everett will drive key commercialization activities at Cognito in conjunction with the leadership team.
By Cognito Therapeutics · Via Business Wire · May 18, 2021

Cognito Therapeutics, a clinical-stage company leading the development of a new class of disease-modifying digital therapeutics to treat neurodegenerative disorders including Alzheimer’s disease, announced today the appointment of Jonathan Lieber as Chief Financial Officer. Mr. Lieber will be responsible for all investor relations, finance and administrative functions, and will lead the Company’s future financing efforts to support the company’s growth initiatives.
By Cognito Therapeutics · Via Business Wire · May 5, 2021
