Articles from SetPoint Medical

SetPoint Medical, a clinical-stage healthcare company dedicated to people living with chronic autoimmune diseases, today announced that the European Alliance of Associations for Rheumatology (EULAR) 2025 Congress selected its RESET-RA study as a recipient of the EULAR Abstract Award in the Clinical Research category. SetPoint was awarded for abstract OP0190 titled, “Neuroimmune Modulation for Treatment of Rheumatoid Arthritis in Adults with Inadequate Response or Intolerance to Biological or Targeted Synthetic DMARDs: Results at 12 and 24 weeks from a Randomized, Sham-Controlled, Double-Blind Study (RESET-RA Study).”
By SetPoint Medical · Via Business Wire · June 11, 2025

SetPoint Medical, a clinical-stage healthcare company dedicated to people living with chronic autoimmune diseases, today announced the appointment of Tyler Binney to its Board of Directors. With a career built around scaling disruptive MedTech platforms, Binney brings over two decades of commercial leadership experience that will be instrumental to SetPoint’s mission to deliver a new treatment alternative to patients with autoimmune conditions, including rheumatoid arthritis (RA).
By SetPoint Medical · Via Business Wire · June 4, 2025

SetPoint Medical, a clinical-stage healthcare company dedicated to people living with chronic autoimmune diseases, today announced that it has filed its premarket approval (PMA) application to the U.S. Food and Drug Administration (FDA) for the SetPoint System, a potential first-of-its-kind neuroimmune modulation device for adults living with moderate-to-severe rheumatoid arthritis (RA) who are incomplete responders or are intolerant to biologic or targeted synthetic disease-modifying anti-rheumatic drugs (DMARDs).
By SetPoint Medical · Via Business Wire · December 9, 2024
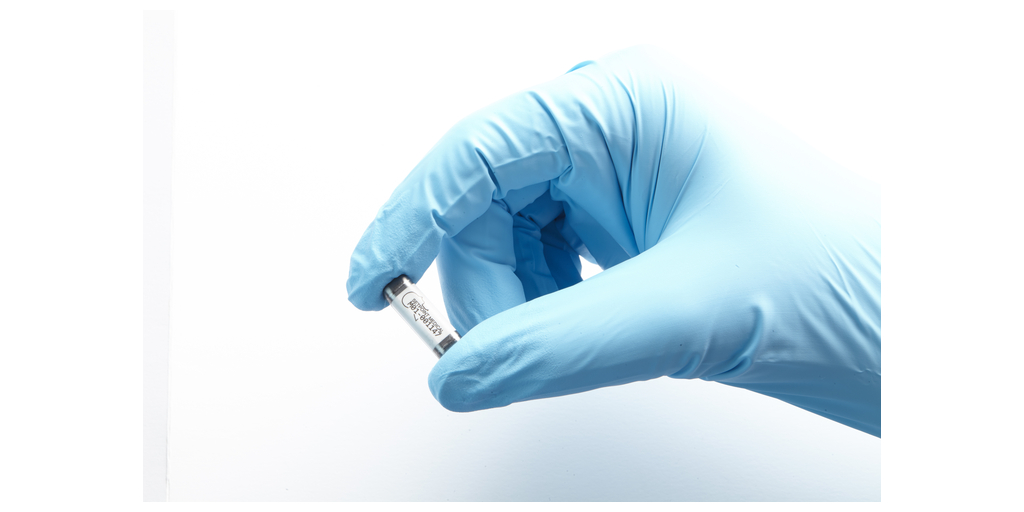
SetPoint Medical, a clinical-stage healthcare company dedicated to people living with chronic autoimmune diseases, today announced late-breaking data from the RESET-RA study presented at the American College of Rheumatology (ACR) Convergence 2024, the largest global gathering of rheumatologists. New clinical trial results support the SetPoint System's potential as a first-of-its-kind neuroimmune modulation treatment for adults living with moderate-to-severe rheumatoid arthritis (RA) who are incomplete responders or intolerant to at least one biologic or targeted synthetic disease-modifying anti-rheumatic drugs (b/tsDMARDs).
By SetPoint Medical · Via Business Wire · November 18, 2024

SetPoint Medical, a clinical-stage healthcare company dedicated to people living with chronic autoimmune diseases, today announced that it has received Investigational Device Exemption (IDE) approval from the U.S. Food and Drug Administration (FDA) to study its proprietary neuroimmune modulation platform in people living with relapsing-remitting multiple sclerosis (RRMS).
By SetPoint Medical · Via Business Wire · October 2, 2024

SetPoint Medical, a clinical-stage healthcare company dedicated to people living with chronic autoimmune diseases, today announced positive topline results from the landmark RESET-RA clinical study. RESET-RA is evaluating the SetPoint System as a potential first-of-its-kind neuroimmune modulation treatment for adults living with moderate-to-severe rheumatoid arthritis (RA) who are incomplete responders or are intolerant to biologic or targeted synthetic disease-modifying anti-rheumatic drugs (DMARDs).
By SetPoint Medical · Via Business Wire · July 10, 2024

SetPoint Medical, a clinical-stage healthcare company dedicated to patients with chronic autoimmune diseases, announced that it has been accepted into the Total Product Life Cycle (TPLC) Advisory Program (TAP) Pilot from the U.S. Food and Drug Administration (FDA) for the development of its novel neuroimmune modulation platform for people living with relapsing-remitting multiple sclerosis (RRMS).
By SetPoint Medical · Via Business Wire · March 21, 2024

SetPoint Medical, a clinical-stage healthcare company dedicated to patients with chronic autoimmune diseases, announced that it has received Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) for the use of its novel neuroimmune modulation platform for people living with relapsing-remitting multiple sclerosis (RRMS). Breakthrough Device Designation will enable interactive communication and priority regulatory review with the FDA, and support reimbursement and patient access upon FDA approval of SetPoint’s technology.
By SetPoint Medical · Via Business Wire · March 13, 2024

SetPoint Medical, a clinical-stage healthcare company dedicated to patients with chronic autoimmune diseases, today announced the publication of data from a multicenter European clinical trial evaluating the safety and efficacy of neuroimmune modulation in patients with treatment-refractory Crohn’s disease in the Journal of Crohn’s and Colitis. The pilot study demonstrated that neuroimmune modulation via vagus nerve stimulation resulted in a clinically meaningful reduction in disease activity and improved quality of life.
By SetPoint Medical · Via Business Wire · October 3, 2023

SetPoint Medical, a clinical-stage healthcare company dedicated to patients with chronic autoimmune diseases, today announced the appointment of Rohan Seth as Chief Financial Officer (CFO).
By SetPoint Medical · Via Business Wire · June 12, 2023

SetPoint Medical, a clinical-stage healthcare company dedicated to patients with chronic autoimmune diseases, today announced a senior secured term loan facility of up to $65 million from Runway Growth Capital LLC (“Runway”), a leading provider of growth loans to both venture and non-venture backed companies. The loan is an expansion of an existing credit facility with Runway. The company recently announced an $80 million equity financing to advance its novel platform for the treatment of autoimmune disease.
By SetPoint Medical · Via Business Wire · January 19, 2023

SetPoint Medical, a clinical-stage healthcare company dedicated to patients with chronic autoimmune diseases, today announced an $80 million preferred stock financing co-led by new investors Norwest Venture Partners and Viking Global Investors. The company is developing its novel platform for the treatment of chronic, inflammation-mediated autoimmune diseases and is initially focused on a potentially less immunosuppressive option for the treatment of rheumatoid arthritis (RA).
By SetPoint Medical · Via Business Wire · January 19, 2023

SetPoint Medical, a clinical-stage healthcare company dedicated to patients with chronic autoimmune diseases, announced the enrollment of the first participant in Stage 2 of the company's RESET-RA pivotal study. The RESET-RA study is evaluating SetPoint Medical's investigational platform technology for treatment of rheumatoid arthritis (RA) using vagus nerve stimulation to activate innate anti-inflammatory pathways. The company recently announced FDA approval for continuation to Stage 2 of the study based on outcomes from an interim data analysis.
By SetPoint Medical · Via Business Wire · October 10, 2022

SetPoint Medical, a clinical-stage healthcare company dedicated to patients with chronic autoimmune disease, announced U.S. Food and Drug Administration (FDA) approval for continuation of the company’s RESET-RA study. The study, which received an Investigational Device Exemption (IDE) from the FDA to evaluate SetPoint Medical’s proprietary therapeutic platform in patients with rheumatoid arthritis (RA), was initiated in January 2021.
By SetPoint Medical · Via Business Wire · September 13, 2022
