Articles from Thermo Fisher

Thermo Fisher Scientific Inc., the world leader in serving science, introduced today new lines of floor-model centrifuges to provide more sustainable solutions without compromising performance and sample security. The Thermo Scientific™ Cryofuge™, Thermo Scientific™ BIOS and Thermo Scientific™ LYNX centrifuges are the first floor-model centrifuges that feature natural refrigerant cooling systems compliant with European Union (E.U.) and U.S. Environmental Protection Agency (EPA) F-gas regulations. Regulatory bodies in the E.U. and U.S. are implementing regulations to discontinue fluorinated gases as refrigerants, and centrifuge manufacturers must comply to the new prohibition timelines.
By Thermo Fisher · Via Business Wire · March 31, 2025

Thermo Fisher Scientific Inc., the world leader in serving science, has announced a Technology Alliance Agreement with the Chan Zuckerberg Institute for Advanced Biological Imaging (CZ Imaging Institute). This agreement aims to develop new technologies to enable researchers to better visualize human cells, significantly advancing scientific research and discovery.
By Thermo Fisher · Via Business Wire · March 27, 2025

Thermo Fisher Scientific Inc., the world leader in serving science, today announced the launch of the CorEvitas Systemic Lupus Erythematosus (SLE) Registry. The multi-center, prospective registry leverages the CorEvitas rheumatology physician network and addresses a critical unmet need for collecting robust, objective real-world data about this chronic and devastating autoimmune disease, in which the immune system attacks healthy tissue and can cause damage to patients’ skin, joints, blood and internal organs, including the kidneys, heart, brain and lungs. The registry will leverage clinician and patient insights to address key questions about the safety and effectiveness of available and future treatments for SLE, the most common type of lupus.
By Thermo Fisher · Via Business Wire · February 26, 2025

Thermo Fisher Scientific Inc., the world leader in serving science, today introduced the Invitrogen™ EVOS™ S1000 Spatial Imaging System. This advanced system addresses the limitations of current fluorescent microscopy technologies by enabling researchers to generate a multiplexed high-quality image for multiple samples within several hours, thereby lowering the barrier to entry into spatial tissue proteomics.
By Thermo Fisher · Via Business Wire · February 12, 2025

Thermo Fisher Scientific Inc., the world leader in serving science, today announced the launch of the international CorEvitas Adolescent Alopecia Areata (AA) Registry, addressing a critical unmet need for real-world, adolescent-specific evidence and data related to this autoimmune disease, which causes patchy or complete hair loss on the scalp and other areas of the body. Data collected will enable research to help better understand the burden of disease for AA patients, as well as the real-world effectiveness and safety of newly approved treatments.
By Thermo Fisher · Via Business Wire · February 5, 2025

Thermo Fisher Scientific, the world leader in serving science, today announced the introduction of Olink® Reveal, setting a new standard for affordable, high-plex proteomics that enables researchers to identify circulating biomarkers for a range of applications with reduced cost and set up requirements.
By Thermo Fisher · Via Business Wire · January 27, 2025

Thermo Fisher Scientific Inc., the world leader in serving science, today unveiled the Gibco™ CTS™ Detachable Dynabeads™ CD4 and CTS Detachable Dynabeads CD8 (CTS Detachable Dynabeads)*. These latest products expand on Thermo Fisher’s CTS Detachable Dynabeads platform, which represents a new generation of cell therapy isolation and/or activation products that prioritize cell quality while also creating greater workflow control. Ultimately, these products can help customers maximize the potential of their therapies to save more lives.
By Thermo Fisher · Via Business Wire · December 4, 2024

Thermo Fisher Scientific, the world leader in serving science, has received approval from the U.S. Food and Drug Administration (FDA) for its Ion Torrent™ Oncomine™ Dx Target Test as a companion diagnostic (CDx) to identify patients eligible for treatment with Servier Pharmaceuticals, LLC’s VORANIGO® (vorasidenib) tablets. VORANIGO is an isocitrate dehydrogenase-1 (IDH1) and isocitrate dehydrogenase-2 (IDH2) inhibitor indicated for the treatment of adult and pediatric patients 12 years and older with Grade 2 astrocytoma or oligodendroglioma with a susceptible IDH1 or IDH2 mutation following surgery including biopsy, sub-total resection or gross total resection. As the first targeted therapy for Grade 2 IDH-mutant glioma, VORANIGO provides a new care path for patients with extremely limited treatment options.
By Thermo Fisher · Via Business Wire · October 21, 2024

Thermo Fisher Scientific Inc., the world leader in serving science, introduced a groundbreaking transmission electron microscope to a packed room of attendees at the recent European Microscopy Congress 2024 in Copenhagen, Denmark. As a fully integrated multimodal analytical solution, the Thermo Scientific™ Iliad™ (Scanning) Transmission Electron Microscope, (S)TEM, gives scientific pioneers deeper insights about the chemical nature of the most sophisticated modern materials, down to the atomic level.
By Thermo Fisher · Via Business Wire · October 14, 2024

Thermo Fisher Scientific Inc., the world leader in serving science, today introduced more sustainable packaging for 125,000 of its Invitrogen™ antibodies. Transitioning from cold-chain packaging to ambient temperature shipping from distribution center to customer reduces package material mass, improves the customer experience and supports the company’s global sustainability efforts.
By Thermo Fisher · Via Business Wire · October 8, 2024

Thermo Fisher Scientific Inc., the world leader in serving science, will showcase its latest innovations enabling the molecule-to-medicine journey and host a series of sessions that feature industry developments during CPHI Milan 2024, Oct. 8-10, in Milan, Italy. Leaders and experts from the company will be present at conference booth #7B18 to discuss how Thermo Fisher is supporting biotechnology and pharmaceutical companies across indications, modalities, sizes and stages of the drug development journey, demonstrating the company’s global momentum.
By Thermo Fisher · Via Business Wire · October 7, 2024
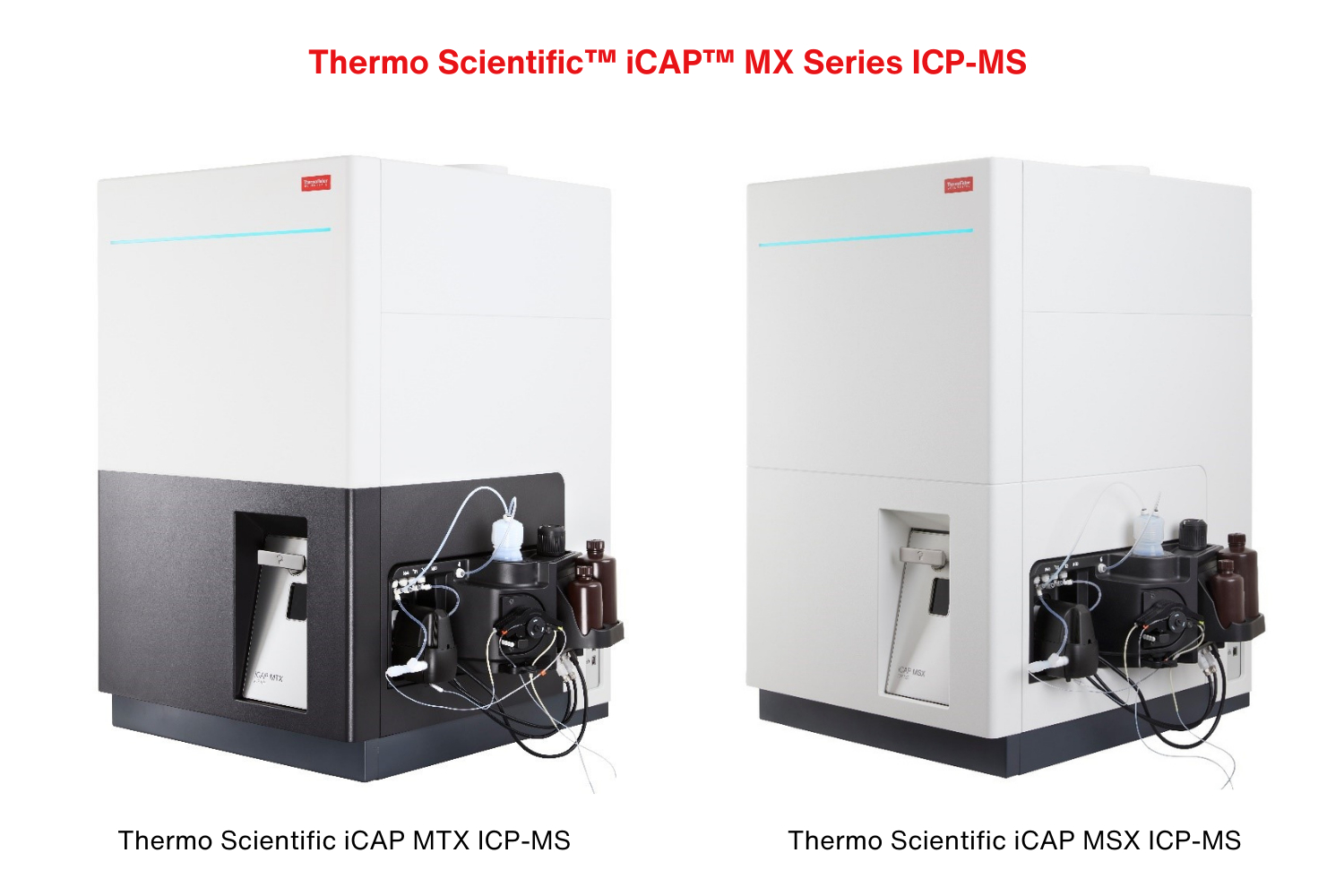
Thermo Fisher Scientific Inc. today launched the Thermo Scientific™ iCAP™ MX Series ICP-MS to simplify trace element analysis with inductively coupled plasma mass spectrometry (ICP-MS). The launch includes a new single quadrupole Thermo Scientific iCAP MSX ICP-MS and triple quadrupole Thermo Scientific iCAP MTX ICP-MS designed for environmental, food, industrial and research labs to analyze routine and challenging trace elements to detect and mitigate harmful substances.
By Thermo Fisher · Via Business Wire · October 1, 2024

Thermo Fisher Scientific Inc., the world leader in serving science, today announced the launch of the international CorEvitas Adolescent Atopic Dermatitis (AD) Registry, designed to study novel treatments for the most common inflammatory skin condition in adolescent patients.
By Thermo Fisher · Via Business Wire · September 30, 2024

To provide flexible and innovative solutions for early development challenges commonly faced by biotech and pharmaceutical companies, Thermo Fisher Scientific Inc. is expanding its oral solid dose (OSD) footprint with a $22-million total investment since 2021 in its Cincinnati, Ohio, and Bend, Ore. sites. Together, these expansions will enable research and development (R&D), manufacturing and testing of OSD drug formulations – doubling the Bend site’s existing footprint and continuing a multi-year investment plan in Cincinnati – to bolster capabilities across the company’s global Contract Development and Manufacturing Organization (CDMO) and Contract Research Organization (CRO) network.
By Thermo Fisher · Via Business Wire · September 26, 2024

The PPD™ clinical research business of Thermo Fisher Scientific, the world leader in serving science, announced today the expansion of its global laboratory services with a new bioanalytical lab in GoCo Health Innovation City in Gothenburg, Sweden, that will serve pharmaceutical and biotech customers with advanced laboratory services and leading-edge instrumentation across all phases of pharmaceutical development to help deliver life-changing medicines to patients worldwide.
By Thermo Fisher · Via Business Wire · September 18, 2024

At the 36th annual European Congress of Pathology in Florence, Italy, Thermo Fisher Scientific, the world leader serving science, announced the latest recipients of the Oncomine Clinical Research Grant, designed to support emerging research on molecular profiling in oncology and to help democratize the future of precision medicine. Now in its fourth year, the grant recognizes research from Tata Memorial Centre in India; Leiden University Center in The Netherlands; Western University in Canada; and Fred Hutch Cancer Center in the U.S.
By Thermo Fisher · Via Business Wire · September 11, 2024

Thermo Fisher Scientific Inc., the world leader in serving science, has received three R&D 100 Awards for innovations that aid researchers’ and scientists’ work in a variety of life science applications, including proteomics, bioproduction and small-molecule analysis. Notably, the Thermo Scientific™ Orbitrap™ Astral™ Mass Spectrometer (MS) won gold in the Market Disruptor special recognition category as one of the most significant advancements in mass spectrometry in 15 years.
By Thermo Fisher · Via Business Wire · August 15, 2024

The PPD clinical research business of Thermo Fisher Scientific, the world leader in serving science, has been honored for industry leadership in digital services by Information Services Group (ISG), a leading global technology research and advisory firm. The business was recognized as an ISG Provider Lens Leader for its digital transformation services in clinical development and patient engagement, as well as pharmacovigilance and regulatory affairs.
By Thermo Fisher · Via Business Wire · August 14, 2024

Thermo Fisher Scientific Inc., the world leader in serving science, today announced that its SeCore™ CDx HLA A Sequencing System has been granted 510(k) clearance by the United States Food and Drug Administration (FDA) for use as a companion diagnostic with TECELRA® (afamitresgene autoleucel), Adaptimmune’s newly approved T-cell receptor (TCR) therapy for the treatment of adults with unresectable or metastatic synovial sarcoma who have received prior chemotherapy, are HLA-A*02:01P, -A*02:02P, -A*02:03P, or -A*02:06P positive and whose tumor expresses the MAGE-A4 antigen as determined by FDA-approved or cleared companion diagnostic devices. Synovial sarcoma is a rare, soft tissue cancer that most commonly impacts young adults.1,2
By Thermo Fisher · Via Business Wire · August 6, 2024

Thermo Fisher Scientific, the world leader in serving science, has introduced a new pre-transplant risk assessment assay via its CLIA laboratory in Fishers, Ind., that helps assess risk of early acute rejection in kidney transplant recipients, providing data that may inform a more personalized approach to post-transplant immunosuppression management.
By Thermo Fisher · Via Business Wire · July 30, 2024

Thermo Fisher Scientific Inc., the world leader in serving science, today announced it has been 510k cleared, following US Food and Drug Administration (FDA) requirements, to market its Optilite® Freelite® assays’ claim for the evaluation of monoclonal gammopathy of undetermined significance (MGUS).
By Thermo Fisher · Via Business Wire · July 29, 2024

Thermo Fisher Scientific Inc. will showcase its broad diagnostics portfolio and host a series of customer-led workshops and presentations that highlight industry developments and the company’s latest innovations during the Association for Diagnostics & Laboratory Medicine Conference (ADLM) July 28-Aug. 1, 2024, in Chicago, Ill. The company’s conference booth (#2213) has also been designed as a “Story Tunnel” to lead visitors along a journey of research and clinical solutions impacting patient care across oncology, infectious disease, women’s health, toxicology, allergy and autoimmune diseases, and transplant and protein diagnostics.
By Thermo Fisher · Via Business Wire · July 25, 2024

To support next-generation semiconductor and materials innovation, Thermo Fisher Scientific today announced the opening of its first electron microscopy demo center in Taiwan. This state-of-the-art facility, called a NanoPort, is strategically located in the region’s semiconductor manufacturing hub and designed to meet the area’s growing need for advanced analytical instruments and expertise. The new center is now one of six Thermo Fisher NanoPort facilities worldwide and will partner with semiconductor customers in the region.
By Thermo Fisher · Via Business Wire · July 9, 2024

Thermo Fisher Scientific Inc., the world leader in serving science, is contributing to the sustainability of biologics therapy manufacturing by leveraging plant-based materials, rather than fossil fuel materials, to deliver lower-carbon, biobased films for its single-use technology bioprocessing containers (BPCs). The biobased films build on Thermo Fisher’s existing Aegis™ and CX film offerings that are widely used and validated by customers. As a result, customers can maintain consistency in their BPCs while also realizing a reduction in greenhouse gas (GHG) emissions. By adopting biobased, sustainable films in their BPCs, Thermo Fisher customers can eliminate carbon emissions related to the plastic resin1.
By Thermo Fisher · Via Business Wire · June 28, 2024

The PPD clinical research business of Thermo Fisher Scientific Inc., the world leader in serving science, today announced the expansion of its central laboratory operations in Kentucky dedicated to accelerating pharmaceutical and biotech customers’ delivery of safe, effective medicines to patients.
By Thermo Fisher · Via Business Wire · June 27, 2024

Thermo Fisher Scientific Inc., the world leader in serving science, today introduced the Thermo Scientific™ KingFisher™ PlasmidPro Maxi Processor* (PlasmidPro), the only fully automated maxi-scale plasmid DNA (pDNA) purification system. PlasmidPro enables innovation at scale, providing complete automation across mini and maxi scale purification and delivering high-purity plasmid without manual column preparation and intervention. This is the latest addition to the Thermo Scientific KingFisher instrument portfolio which offers a wide range of plasmid DNA extraction products to help drive efficiency and consistency.
By Thermo Fisher · Via Business Wire · June 25, 2024

The PPD clinical research business of Thermo Fisher Scientific Inc., the world leader in serving science, today unveiled a new clinical research laboratory building at its good manufacturing practices (GMP) lab in Middleton, Wisconsin. The new 72,500-square-foot building increases the site’s chemistry, manufacturing and control (CMC) analytical capabilities as part of its clinical development and laboratory services focused on improving health by helping customers deliver life-changing medicines.
By Thermo Fisher · Via Business Wire · June 18, 2024

The PPD clinical research business of Thermo Fisher Scientific Inc., the world leader in serving science, is embracing the theme of the 60th annual Drug Information Association (DIA) annual meeting today in San Diego – “Charting New Horizons.” The same day the business earns a CRO Leadership Award for the 13th consecutive year, it’s also hosting a DIA innovation panel for customers on accelerating drug development with real-world data and technology.
By Thermo Fisher · Via Business Wire · June 17, 2024

To support the future implementation of workflow automation in cell therapy production, Thermo Fisher Scientific introduces the Thermo Scientific™ Heracell™ VIOS™ 250i AxD CO2 Incubators. These first-of-their-kind CO2 incubators are designed for integration into automated and modular laboratories. The VIOS family of incubators are known for optimal cell growth conditions and minimal contamination risk. Now adding the innovation of patent-pending automated door control, the Heracell VIOS AxD CO2 incubator opens automatically when integrated into a centralized lab automation platform, allowing vessel loading and unloading through robotic control and supporting continuous cell therapy production processes.
By Thermo Fisher · Via Business Wire · June 12, 2024

To help accelerate the promise of precision medicine, Thermo Fisher Scientific today unveiled its new solutions accelerating research workflows at the annual American Society for Mass Spectrometry (ASMS) Conference on Mass Spectrometry and Allied Topics, in Anaheim, California. The new mass spectrometry and chromatography instruments and software solutions enable customers to unlock deeper analytical insights with built-for-purpose flexibility, improving productivity, efficiency, and accelerating translational research workflows.
By Thermo Fisher · Via Business Wire · June 3, 2024

Thermo Fisher Scientific Inc., the world leader in serving science, has opened a new clinical and commercial ultra-cold facility in the EU, expanding its clinical trial network in Europe to help accelerate the development of advanced therapies. The new current good manufacturing practice (cGMP) facility in Bleiswijk, Netherlands provides pharma and biopharma customers tailored, end-to-end support throughout the clinical supply chain for high-value therapies, including cell and gene therapies, biologics, antibodies and vaccines.
By Thermo Fisher · Via Business Wire · June 3, 2024

Thermo Fisher Scientific Inc., the world leader in serving science, today introduced the Applied Biosystems™ Axiom™ BloodGenomiX™ Array* and Software, a first-of-its-kind solution for more precise blood genotyping in clinical research. The new array detects most extended and rare blood groups, tissue (HLA) and platelet (HPA) types in a single, high-throughput assay, supporting future advancements in donor blood matching for extended phenotypes.
By Thermo Fisher · Via Business Wire · May 15, 2024

For organ transplant recipients, life post-transplant involves a delicate balance between invasive monitoring and immunosuppression to reduce the risk of rejection, both of which may carry complications. Now, a simple, urine-based assay that detects the CXCL10 chemokine may make care more convenient and less invasive for the nearly 250,000 Americans living with a kidney transplant.
By Thermo Fisher · Via Business Wire · May 2, 2024
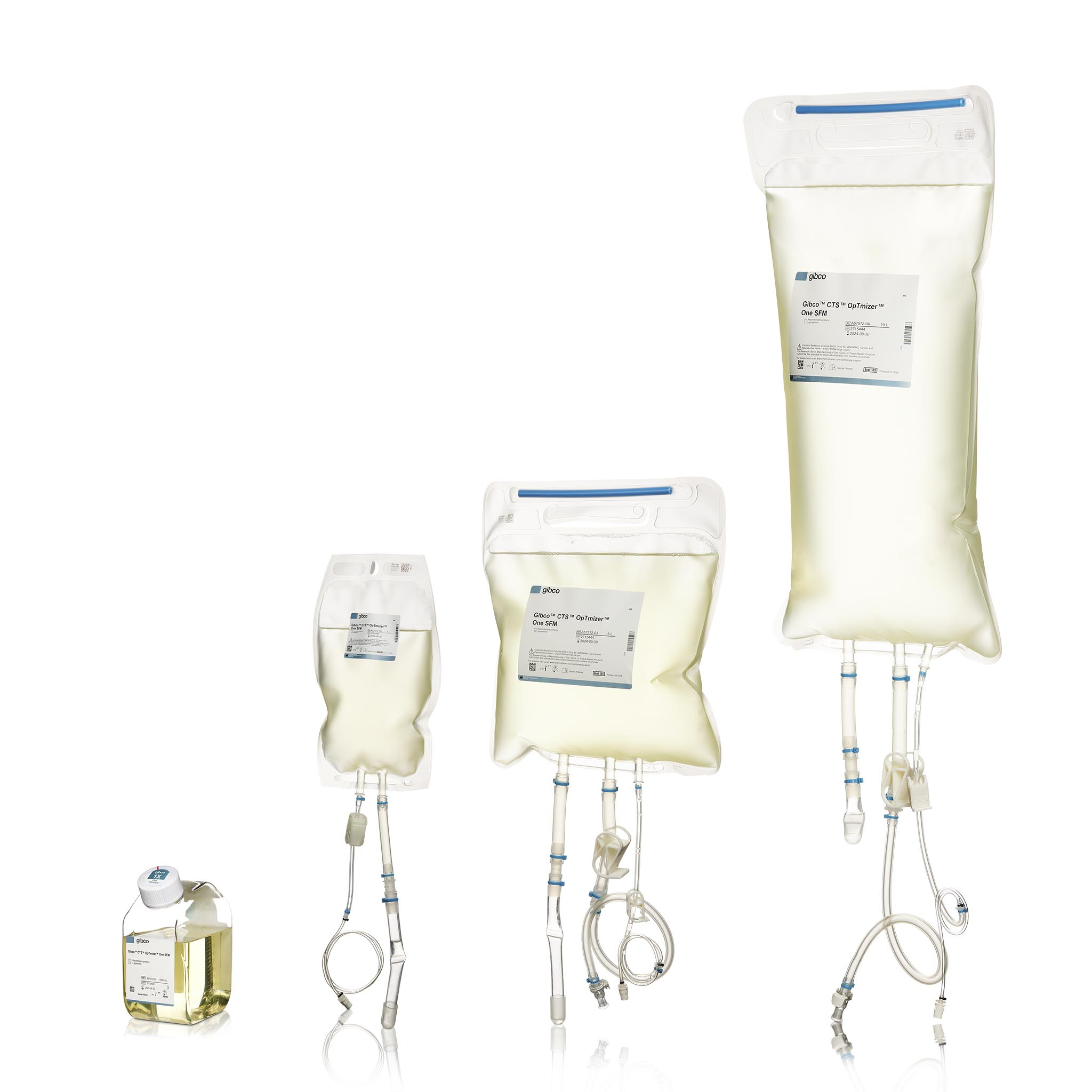
Thermo Fisher Scientific Inc., the world leader in serving science, has introduced the Gibco™ CTS™ OpTmizer™ One Serum-Free Medium (CTS OpTmizer One SFM)*, a novel animal origin-free (AOF) formulation designed specifically for clinical and commercial cell therapy manufacturing to deliver increased scalability and performance of T cell expansion. As a result, cell therapy manufacturers can use CTS OpTmizer One SFM to help optimize their workflows and get T cell therapies to patients faster.
By Thermo Fisher · Via Business Wire · April 11, 2024

Analytica 2024 - Thermo Fisher Scientific, Inc. is debuting its recently launched innovative products, hosting educational presentations and showcasing live lab demos for customers working in laboratory technology, analysis, biotechnology, and environmental quality during the 2024 Analytica Conference.
By Thermo Fisher · Via Business Wire · April 9, 2024

Building on 80 years of expertise engineering cold storage lab equipment, Thermo Fisher Scientific today introduces its newest line of high performance, ultra-low temperature (ULT) freezers. With enhancements to performance, user experience and energy efficiency, the Thermo Scientific™ TSX™ Universal Series ULT Freezers seamlessly adapt to scientists’ workflows across a variety of lab settings, marking a new era in performance, reliability, and sustainability.
By Thermo Fisher · Via Business Wire · April 8, 2024

Thermo Fisher Scientific Inc., the world leader in serving science, today announced the launch of a new CorEvitas syndicated clinical registry in generalized pustular psoriasis (GPP). This registry, which is open to enrollment, is CorEvitas’ 10th syndicated disease registry and addresses an unmet need for real-world evidence (RWE) related to the clinical and patient-reported outcomes of patients with GPP.
By Thermo Fisher · Via Business Wire · March 8, 2024

Today, Thermo Fisher Scientific announced the latest recipients of the Oncomine Clinical Research Grant, recognizing research from the Melanoma Institute Australia, the University of Turin in Italy and Atrium Health Wake Forest Baptist Medical Center. The grant program, now in its seventh round of awardees, supports investigator-initiated studies with the aim to increase high-quality molecular profiling in oncology and to help democratize precision medicine.
By Thermo Fisher · Via Business Wire · February 20, 2024

For the 11th consecutive year, Thermo Fisher Scientific’s pharma and biopharma customers recognized its contract manufacturing excellence through the annual CDMO Leadership awards sponsored by Outsourced Pharma and Life Science Connect. Thermo Fisher received five awards for capabilities, compatibility, expertise, and quality and reliability from three customer groups: big pharma, small pharma, and both.
By Thermo Fisher · Via Business Wire · February 16, 2024

To support a wider range of ion chromatography analysis with one instrument, Thermo Fisher Scientific Inc., today launched the Thermo Scientific™ Dionex™ Inuvion™ Ion Chromatography (IC) system, helping to make ion analysis simpler and more intuitive for labs of all sizes. The new analytical instrument is designed to be easily reconfigurable, providing those who require determination of ionic and small polar compounds with a one stop shop for consistent, reliable ion analysis.
By Thermo Fisher · Via Business Wire · February 14, 2024

The PPD clinical research business of Thermo Fisher Scientific Inc., the world leader in serving science, is adding mycoplasma and additional biosafety testing capabilities to its expanding portfolio of services at its good manufacturing practices (GMP) lab in Middleton, Wisconsin. This new service, offered by the analytical testing service of the clinical research business, ensures biopharmaceutical products are free of contaminants, helping customers deliver safe medicines for patients.
By Thermo Fisher · Via Business Wire · February 13, 2024

Thermo Fisher Scientific, the world leader in serving science, has received Good Manufacturing Practice (GMP) approval from the Italian Medicines Agency (AIFA) allowing the company to manufacture RNA-based products at its Monza, Italy site. The approval and associated certification support increased accessibility to novel therapies for patients with difficult to treat conditions, marking a significant achievement within the Thermo Fisher Scientific network and for Italy as a whole.
By Thermo Fisher · Via Business Wire · January 18, 2024
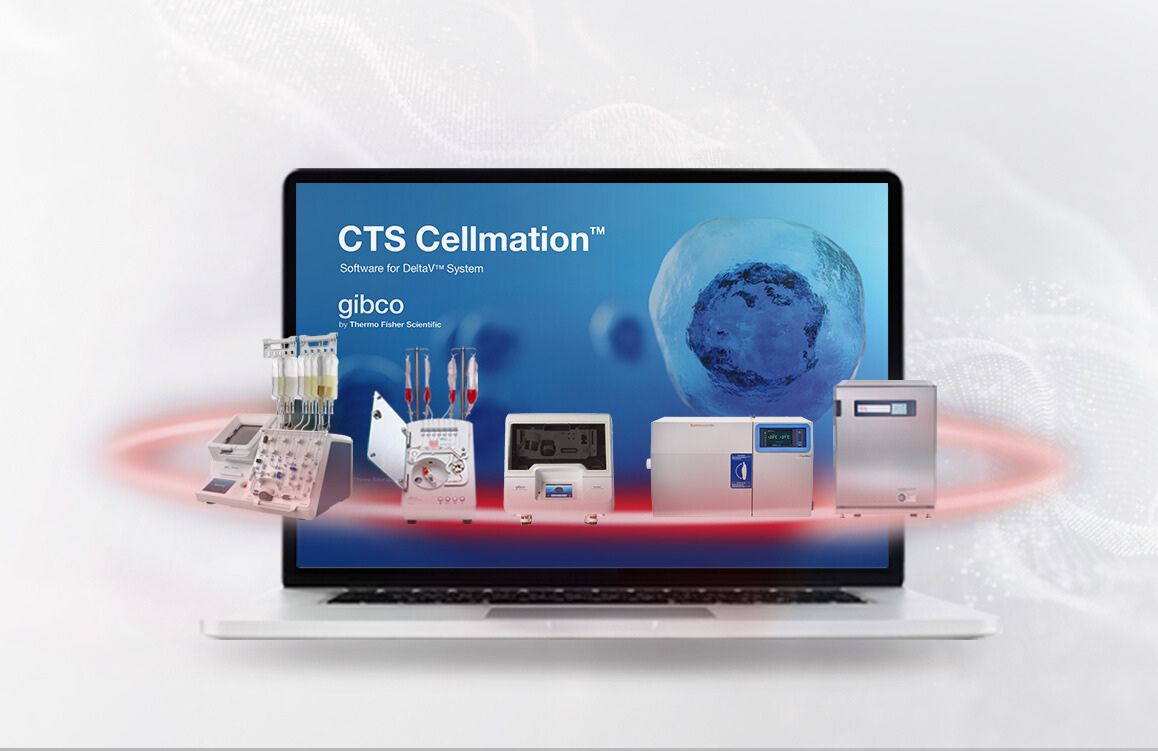
To optimize clinical manufacturing processes for innovators developing breakthrough cell therapies, Thermo Fisher Scientific today announced the launch of the Gibco™ Cell Therapy Systems (CTS™) Cellmation™ Software, a first-of-its-kind automation solution designed to connect and integrate workflows across multiple Thermo Fisher Scientific cell therapy instruments while enabling cGMP compliance. This new offering can help eliminate the need for costly custom software projects and extensive validation, saving valuable time and resources during the cell therapy manufacturing process and helping to deliver these promising curative therapies more quickly and safely to patients.
By Thermo Fisher · Via Business Wire · January 17, 2024

Earlier this year, scientists unveiled the first draft of the pangenome, based on full genetic blueprints from 47 people around the world to create a more diverse representation of the human genome1. To more accurately account for this richer picture of human genomic variation, Thermo Fisher Scientific has launched the new Axiom™ PangenomiX Array*, its largest and most ethnically diverse array to date, offering optimal genetic coverage for population scale disease studies and pharmacogenomic research.
By Thermo Fisher · Via Business Wire · January 4, 2024

The PPD clinical research business of Thermo Fisher Scientific Inc., the world leader in serving science, has been selected by the Biomedical Advanced Research and Development Authority (BARDA) to implement the first BARDA-supported Phase II platform clinical trial to investigate multiple therapeutic options for the treatment of acute respiratory distress syndrome (ARDS). BARDA is part of the Administration for Strategic Preparedness and Response (ASPR) within the U.S. Department of Health and Human Services (HHS).
By Thermo Fisher · Via Business Wire · December 21, 2023

Thermo Fisher Scientific Inc., the world leader in serving science, today announced the launch of the Thermo Scientific™ KingFisher™ Apex™ Dx, an automated nucleic acid purification instrument, and Applied Biosystems™ MagMAX™ Dx Viral/Pathogen NA Isolation Kit for the isolation and purification of viral and bacterial pathogens from respiratory biological specimens. Together these products provide laboratories with an in vitro diagnostic (IVD) and in vitro diagnostic regulation (IVD-R) approved automated sample preparation solutions for increased confidence in downstream results.
By Thermo Fisher · Via Business Wire · December 11, 2023

Thermo Fisher Scientific Inc., the world leader in serving science, and Project HOPE, a leading global health and humanitarian organization, today announced a new partnership to improve the wellbeing and treatment outcomes of adolescents and young persons living with HIV in Nigeria, the country with the second largest HIV epidemic worldwide. Nigeria currently accounts for nearly two million individuals living with HIV, contributing to approximately two-thirds of new HIV infections in West and Central Africa.
By Thermo Fisher · Via Business Wire · December 1, 2023

Thermo Fisher Scientific, the world leader in serving science, today announced the opening of its Battery Customer Experience Center in Seoul, South Korea. The advanced facility will assist battery manufacturers in driving innovative solutions that support the United Nations Sustainable Development Goal of providing access to affordable, reliable, sustainable and modern energy for all people by 2030. It also will serve as a global hub for clean energy and battery manufacturing applications, product information and education. The new center will accelerate the development of the next generation of environmentally friendly energy solutions that reduce the world’s reliance on fossil fuels, in line with Thermo Fisher’s company mission to enable its customers to make the world healthier, cleaner and safer.
By Thermo Fisher · Via Business Wire · November 13, 2023

Thermo Fisher Scientific, the world leader in serving science, has introduced the Thermo Scientific™ Meridian™ EX System— an electron-beam-based failure analysis solution designed to enable precise fault localization on advanced semiconductor logic technologies.
By Thermo Fisher · Via Business Wire · October 31, 2023

To support emerging precision therapies and improve patient outcomes by increasing access to reliable genomic testing needed to match patients with targeted cancer treatments, Thermo Fisher Scientific today announced a companion diagnostic (CDx) partnership with Boehringer Ingelheim. Through this collaboration, the companies will work to develop CDx tests to help identify patients with non-small cell lung cancer (NSCLC) with specific genomic mutations.
By Thermo Fisher · Via Business Wire · October 19, 2023

Thermo Fisher Scientific, the world leader in serving science, today expanded its manufacturing capacity in St. Louis to support biologic therapies for diseases ranging from cancers to auto immune conditions to rare genetic disorders.
By Thermo Fisher · Via Business Wire · October 5, 2023

Thermo Fisher Scientific Inc., the world leader in serving science, has been recognized by the R&D 100 Awards, honoring the top 100 revolutionary products in science and technology from around the world, for three unique innovations that aid researchers’ and scientists’ work in a variety of life science applications, including bioproduction and small-molecule analysis.
By Thermo Fisher · Via Business Wire · September 8, 2023

Thermo Fisher Scientific Inc., the world leader in serving science, today announced the Gibco CTS Detachable Dynabeads (CTS Detachable Dynabeads)*, its next-generation platform of Dynabeads with the first active release mechanism of its kind for clinical trial and commercial manufacturing use.
By Thermo Fisher · Via Business Wire · September 6, 2023

Thermo Fisher Scientific, the world leader in serving science, today announced the commercial launch of the EXENT® Solution, after receiving IVDR certification*. The EXENT solution is a fully integrated and automated mass spectrometry system designed to transform diagnosis and assessment for patients with monoclonal gammopathies, including multiple myeloma which, according to the World Health Organization in 2020, is the second most prevalent blood cancer worldwide[1]. The EXENT Solution is now commercially available in the following countries: Belgium, France, Germany, Italy, the Netherlands, Spain and United Kingdom.
By Thermo Fisher · Via Business Wire · August 24, 2023

Thermo Fisher Scientific, the world leader in serving science, today announced the launch of a new chromosomal microarray designed to improve cytogenetic research lab productivity, efficiency and profitability with an industry-leading two-day turnaround time. Providing insights on chromosomal variants for a wide range of prenatal, postnatal and oncology research applications, the Applied Biosystems™ CytoScan™ HD Accel array analyzes the whole human genome and provides improved coverage in more than 5,000 critical genome regions.
By Thermo Fisher · Via Business Wire · August 22, 2023

The PPD clinical research business of Thermo Fisher Scientific Inc., the world leader in serving science, has been granted a five-year award to provide a Transplantation Statistical and Clinical Coordinating Center for the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health in the United States. This center will offer a broad range of support services critical to the design, development, execution and analysis of NIAID transplantation clinical trials and research.
By Thermo Fisher · Via Business Wire · August 10, 2023

Thermo Fisher Scientific, the world leader in serving science, announced today a new line of Thermo Scientific TM TSG Series Refrigerators to ensure the safe storage of critical vaccines and pharmaceuticals for laboratory, pharmacy and clinical* environments.
By Thermo Fisher · Via Business Wire · August 8, 2023

To extend the benefits of genomic testing research for reproductive health labs, Thermo Fisher Scientific today launched two new next-generation sequencing-based options to support preimplantation genetic testing-aneuploidy (PGT-A) used commonly to inform in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) research. The Ion ReproSeq PGT-A Kit* and the Ion AmpliSeq Polyploidy Kit* mark the first research use reproductive health assays available on the Ion Torrent Genexus Integrated Sequencer*, delivering complete workflows from sample to result for aneuploidy analysis.
By Thermo Fisher · Via Business Wire · July 17, 2023

To address challenges with error-prone, manual molecular testing workflows, Thermo Fisher Scientific today has introduced Diomni Enterprise Software, helping streamline routine diagnostics testing for standardization and faster time-to-results. By connecting workflow steps within one interface, Diomni Enterprise can increase a lab’s ability to dynamically respond to today’s rapidly changing testing environment. The solution can be used by a variety of labs performing routine quantitative polymerase chain reaction (qPCR) diagnostic tests and by developers to increase the speed of innovation for emerging testing solutions.
By Thermo Fisher · Via Business Wire · July 13, 2023

Thermo Fisher Scientific, the world leader in serving science, today announced the launch of the Gibco™ OncoPro™ Tumoroid Culture Medium Kit*, the first commercially available culture medium specifically developed for the expansion of patient-derived tumoroids, or cancer organoids, from multiple cancer indications.
By Thermo Fisher · Via Business Wire · June 28, 2023

Thermo Fisher Scientific, the world leader in serving science, has introduced the Thermo Scientific™ Metrios™ 6 Scanning Transmission Electron Microscope ((S)TEM) — a new-generation, fully automated (S)TEM metrology solution designed to help enhance productivity and deliver data quality assurance for high-volume semiconductor manufacturing.
By Thermo Fisher · Via Business Wire · June 27, 2023

Thermo Fisher Scientific Inc., the world leader in serving science, has completed its acquisition of MarqMetrix, a privately held developer of Raman-based spectroscopy solutions for in-line measurement. The terms of the deal were not disclosed.
By Thermo Fisher · Via Business Wire · June 21, 2023

Thermo Fisher Scientific is showcasing new mass spectrometry and chromatography instruments, software and workflows that enable customers to unlock deeper analytical insights, improve productivity and accelerate biological discovery. The company will highlight these innovations during the annual American Society for Mass Spectrometry (ASMS) Conference on Mass Spectrometry and Allied Topics, from June 3-8, 2023, in Houston, Texas, in booth 700 at the George R. Brown Convention Center and at the Hilton Americas-Houston Lanier Grand Ballroom A, B, C.
By Thermo Fisher · Via Business Wire · June 5, 2023

Thermo Fisher Scientific, the world leader in serving science, has opened a new sterile drug facility in Singapore that will better enable customers to deliver new medicines and vaccines in the Asia-Pacific market. The new facility also marks a significant milestone and investment in pandemic preparedness for Singapore, which is fast emerging as a biomedical hub in the Asia-Pacific region.
By Thermo Fisher · Via Business Wire · May 19, 2023

In support of global initiatives to advance molecular profiling in oncology, Thermo Fisher Scientific today announced the latest recipients of the Oncomine Clinical Research Grant and the opening of its submissions for Spring 2023. Grant recipients are leading the exploration of new and expanded applications for next-generation sequencing (NGS)-based testing so that more cancer patients can access the benefits of precision medicine.
By Thermo Fisher · Via Business Wire · April 17, 2023

Thermo Fisher Scientific, the world leader in serving science, is adding early development work for oral solid dose therapies to its Bourgoin, France site, enabling that location to address customers’ workflow from early drug development through commercial manufacturing and underscoring the company’s commitment to helping clients get medicines to patients sooner.
By Thermo Fisher · Via Business Wire · April 6, 2023

Thermo Fisher Scientific, the world leader in serving science, and Arsenal Biosciences, Inc. (ArsenalBio), a clinical-stage cell therapy company engineering advanced chimeric antigen receptor (CAR)-T cell therapies for solid tumors, today announced an update to our strategic collaboration to further the development of manufacturing processes for new cancer treatments. This research and process development-focused collaboration has enabled ArsenalBio to develop a robust manufacturing process for their next-generation, programmable autologous T cells for the treatment of cancer.
By Thermo Fisher · Via Business Wire · March 29, 2023

The PPD clinical research business of Thermo Fisher Scientific, the world leader in serving science, has been distinguished for industry leadership in digital services by Information Services Group (ISG), a leading global technology research and advisory firm. The business was recognized as an ISG Provider Lens Leader for its clinical development digital transformation services, patient engagement digital transformation services, and pharmacovigilance and regulatory affairs. This marks the third consecutive year the business’ leadership has been acknowledged by ISG.
By Thermo Fisher · Via Business Wire · February 27, 2023

The PPD clinical research business of Thermo Fisher Scientific Inc., the world leader in serving science, has been awarded a five-year contract to provide regulatory affairs support and related services for the Blueprint MedTech (BPMT) program, a new multi-institute/center initiative at the National Institutes of Health (NIH) supporting development of translational neurological devices. The BPMT program is a new NIH incubator with a collaborative reach across 11 NIH institutes, whose goal is to accelerate patient access to safe, effective, cutting-edge medical devices to diagnose and/or treat disorders of the nervous system. During each year of the contract, the BPMT incubator aims to support the development of multiple novel technologies from pharmaceutical, biotechnology and device companies.
By Thermo Fisher · Via Business Wire · February 21, 2023

Thermo Fisher Scientific, the world leader in serving science, today announced the launch of its Applied Biosystems TrueMark STI Select Panel, a polymerase chain reaction (PCR) research use only test designed to detect Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis and Mycoplasma genitalium in one test, as well as RNase P, included as a human internal control.
By Thermo Fisher · Via Business Wire · February 8, 2023

Thermo Fisher Scientific, the world leader in serving science, today announced the launch of its CE-IVD marked Applied Biosystems TaqPath Seq HIV-1 Genotyping Kit, a Sanger sequencing-based assay that examines positive samples of human immunodeficiency virus (HIV) to identify genetic variants that may resist common antiretroviral therapeutics.
By Thermo Fisher · Via Business Wire · January 24, 2023

As part of a global, multiyear agreement, Thermo Fisher Scientific today announced it is working with AstraZeneca to develop a solid tissue and blood-based companion diagnostic (CDx) test for Tagrisso (osimertinib). The CDx will help identify patients with non-small cell lung cancer (NSCLC) who may be eligible for treatment with Tagrisso by identifying tumors that exhibit epidermal growth factor receptor (EGFR) alterations including exon 21 L858R mutations, exon 19 deletions or T790M mutations.
By Thermo Fisher · Via Business Wire · January 24, 2023

As part of its efforts to support emerging biotechnology companies with addressing key challenges of bringing new therapies to market, Thermo Fisher Scientific today announced it has become a founding sponsor of Momentum Labs in Alachua, Fla. Momentum Labs will serve as a collaborative laboratory and office space for growing biotech businesses in the greater Gainesville region. The venture is spearheaded by Concept Companies, a national real estate development firm headquartered in Gainesville.
By Thermo Fisher · Via Business Wire · January 12, 2023

Thermo Fisher Scientific Inc., the world leader in serving science, received R&D 100 Awards from R&D World Magazine for two of its most recently launched analytical instruments. The R&D 100 Awards competition honors the latest innovations in science and technology, identifying the top 100 revolutionary technologies of the past year.
By Thermo Fisher · Via Business Wire · December 15, 2022

Thermo Fisher Scientific, the world leader in serving science, today announced the U.S. Food and Drug Administration (FDA) has granted Emergency Use Authorization (EUA) for its Applied Biosystems TaqPath Monkeypox/Orthopox Virus DNA Kit, a polymerase chain reaction (PCR) test designed to detect non-variola Orthopoxviruses, including monkeypox virus, in approximately three-and-a-half hours.
By Thermo Fisher · Via Business Wire · December 14, 2022
